The prolonged use of heat transfer fluids at high temperature inevitably leads to thermal degradation of the fluid. This process can be slowed by methods such as decreasing the temperature of the operation, which reduces the fluid’s “thermal cracking.” It does not, however, halt the process – it simply slows its rate. So manufacturers need to adopt a proactive approach to monitoring the extent of degradation and managing the removal of byproducts (e.g., “light ends” or flammable vapors) formed during this process. How can your facility actively remove light ends and sustain a normal and safe operation? Let’s look at some tactics for doing so.
P&S Market Research reported in March that the global heat transfer fluid (HTF) market was valued at more than $2.8 billion in 2015 is and projected to grow by 6.8% over the next five years. HTFs are used in a number of manufacturing plants, including food manufacturing facilities. The HTFs used in such processes are normally colorless (i.e., they look like water when new), nontoxic and nonirritating. This is important for the safe handling of the fluid, but as the fluid may potentially come into contact with the food, it also needs to be safe for this use. Hence, food-grade HTFs have a HT-1 certification to show they can be used for “incidental contact with food.”
In addition to its safety factors, this type of fluid is also nonfouling. This means that it forms smaller amounts of carbon when it thermally degrades; this reduces the fouling of internal pipework. HAZTECH Consultants’ Tony Ennis in 2009 described the process of thermal degradation as follows:
“When a heat transfer fluid degrades, it forms a combination of ‘lights’ and ‘heavies.’ The lights are short chain molecules formed by thermal cracking and may also include hydrogen gas. These have the effect of reducing the flashpoint of the HTF as well as being boiled off from the liquid as a flammable vapour.”
How can flammable vapors (light ends) be monitored?
Light ends will accumulate in an HTF and can be detected by laboratory analysis. Indeed, if light ends accumulate, the flash-point temperatures (open and closed) of an HTF will drop. Open flash-point temperature simulates flammable vapors mixing with air and means the vapors can escape to atmosphere. Closed flash-point temperature simulates vapors being enclosed and not mixing with air, which means the most flammable vapors do not escape. In this scenario, the vapors are maintained in the HTF and would better represent the fluid in the HTF system. When an HTF is sampled, it is important that a representative sample of the HTF be obtained. This also means that the fluid needs to be sampled to a closed sampling device that it is not open to air.
Figure 1. An image of a sampling device used to obtain a representative HTF sample.
An example of a closed sampling device is shown in Figure 1. Another important difference between flash-point temperatures is that closed flash-point temperature is lower than the open flash-point temperature, but this difference is dependent on the type of fluid.
The open and closed flash-point temperatures for a virgin mineral-based HTF are approximately 230 degrees and 210 degrees Celsius. During thermal degradation, these will drop, and at certain temperatures an intervention will be required. This is driven by the type of HTF, but for a mineral-based HTF, serious ratings would be considered if open flash-point temperature was ≤150 degrees Celsius and closed flash-point temperature was ≤85 degrees Celsius. Table 1 cites the typical values used to rate the seriousness of test results for a mineral-based HTF with ratings ranging from normal (and receiving a “satisfactory” rating) to thermally degraded (i.e., “serious” and requiring replacement of the HTF).
Table 1. Ratings for closed and open flash point temperatures for a mineral-based HTF.
What interventions can be used to manage flammable vapors?
Figure 2. An image of a permanently installed LERK.
Two ways in which light ends can be removed from a HTF system are batch venting and installation of a light ends removal kit, or LERK (see Figure 2). Batch venting refers to the process of heating the expansion tank and raising the temperature of the circulating HTF. This will vaporize the light ends and hence remove them from the HTF and HTF system. The downside to batch venting, however, is that the increased temperature will increase the rate of oxidation according to the Arrhenius rule and accelerate thermal degradation of the HTF. A nitrogen lance can be used to negate the effect of oxidation, but by its nature, batch venting is an aggressive approach and continued until no further light ends are collected.
In contrast, a LERK is installed and removes light ends continuously via distillation. This means that light ends can be removed while the HTF system is in operation and does not require the header tank to be heated. A LERK also offers the advantage of being able to be installed temporarily or permanently. Once installed, a LERK stops the flammable vapors from building up; this means that the flash point of the fluid is maintained relatively constant.
{pb}
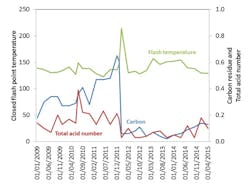
Figure 3 shows data from a customer’s site after a LERK had been installed and shows increases in total acid number (to 0.39 at 7/25/2010) and carbon residue (to 0.65 at 12/6/2011). These changes occurred independently of flash-point temperature. At the high carbon level, flash-point temperature started to increase, which would seem to be explained by high levels of carbon perhaps becoming blocked in the HTF system and representing the importance of condition-based monitoring of HTFs.
Figure 3. Real world data showing stabilisation of closed flash point temperature following installation of a LERK irrespective of changes in carbon residue (indicating thermal cracking) and acidity (indicative of oxidation).
Table 2 compares the batch venting and LERK, highlighting the versatility of a LERK in the short- and long-term control of closed flash-point temperature. It also shows that light ends can effectively be managed using batch venting or a LERK but that long-term control is best achieved with a permanent installation.
Table 2. Things to consider when choosing batch venting or installation of a LERK [4].
Conclusions
Chris Wright is a research scientist with Global Group of Companies and has a B.S. and a Ph.D. from the University of Leeds in the U.K. His research focuses on the use and maintenance of heat transfer fluids in manufacturing and processing, which includes the food, pharmaceutical, specialist chemicals, and solar sectors. Contact him at [email protected].
Flammable vapors can be removed by batch venting or via installation of a LERK, as both have been shown to be effective. The clear advantage of batch venting is that it is cheaper than installing a LERK. In contrast, a LERK is more expensive, but it can be installed temporarily or permanently and removes light ends continuously. Indeed, a research article published in Heat Transfer Engineering (Vol. 37, Issue 15) showed a LERK was still working effectively in a food processing plant more than five years after its initial installation (also see Figure 3).
Unlike with batch venting, a LERK can be built into a system or temporarily plugged in as needed without leading to costly interruptions in production. Lastly, batch venting is quite an aggressive intervention that can ultimately accelerate the degradation of the fluid. This can be avoided by installing a LERK.