The most crucial part of a mechanical seal is the circle where rotary and stationary seal rings touch (see Figure 1). That's where the action occurs. A rubbing, frictional contact simultaneously generates heat and seals fluid. Selecting the right seal rings ensures reliability. They need to be hard, rigid, chemically resistant, thermally conductive, tough and wear resistant. Seal face combinations need to be optimized for the process fluid being sealed.
Figure 1. Single cartridge seal.
Carbon graphite
The most frequently used seal ring material is carbon graphite (see Figure 2). Most seal rings are molded individually. During the process, they undergo a series of changes that increases their hardness and density. Carbon graphite has excellent lubricating qualities and is suitable for a range of temperatures and corrosive environments.
Figure 2. Carbon graphite seal ring parts.
Seal rings of carbon graphite are self-lubricating. Rubbing against the opposite seal, they quickly lay down a microscopically thin, low-friction film of graphite that controls the temperature rise should the interface become dry during operation. But, film development requires the presence of water or other absorbable vapor. Carbon graphite with special impregnants is available for those rare applications that lack vapors such as cryogenic service or operation at high altitudes.
Many grades of carbon graphite are available. A common high-end carbon graphite material contains approximately 80 percent carbon, 20 percent graphite and less than one percent binder. It offers high strength, hardness and modulus of elasticity with a low permeability relative to the other grades. Seal faces with higher graphite content are used in split-seals. On the other hand, lower quality grades have a lower modulus of elasticity and will bend or distort easily under pressure. Also, these lower quality grades may contain impurities that can be attacked by the chemicals the seal is holding back.
Carbon graphite faces are compatible with nearly any chemical or combination of chemicals. However, carbon graphite should never be used with strong acids or oxidizing agents, both of which etch away the seal face surface.
Another concern is carbon blisteringthe formation of small, localized protuberances on the seal face. This condition is associated with the presence of hydrocarbons and cyclical temperature service. The physical mechanism behind blistering is not clear. The most popular explanation is that the carbon substrate absorbs process fluid, which frictional heat then expands, thus producing subsurface pressure and, eventually, a raised spot on the seal face.
Carbon has the lowest modulus of elasticity of any seal ring material, which explains the importance of designing seal rings to accommodate pressure and thermal distortion. Enhanced finite element techniques supported by extensive lab testing allow designers to optimize seal ring geometry to maintain face flatness under varying operating conditions.
Aluminum oxide ceramic
While an aluminum oxide ceramic seal ring exhibits high hardness and excellent chemical resistance, its thermal conductivity is low, which limits its ability to dissipate heat. This is especially problematic with dual cartridge seals, most of which rely on seal ring thermal conductivity to dissipate heat. Using ceramic seal rings raises the operating temperature, which reduces seal reliability. For this reason, ceramic no longer is popular for dual seals.
However, ceramic continues to be an ideal choice for split seal applications (see Figure 3). They are popular on low-pressure, water-based applications that have large amounts of fluid circulating inside the seal gland to provide cooling. When split seals are used on high-pressure applications such as boiler feed pumps, silicon carbide or other higher-performance material replaces ceramic. The 99.5-percent alumina grade is an ideal choice to minimize any concerns of chemical attack from impurities.
Figure 3. Ceramic seal ring parts
Tungsten carbide
Tungsten carbide, another common seal ring material, is made by heating tungsten carbide powder and a metallic binder to 2,700 F (1,500 C) to melt and fuse
the binder. This produces an extremely tough, hard ceramic-metal (cer-met), which has good abrasion resistance and is more dense than other seal ring materials (see Figure 4).
Figure 4. Tungsten carbide seal ring and other parts.
Cobalt and nickel are the two metal binders used. A popular chemical-resistant tungsten carbide uses 6 percent to 10 percent nickel as a binder. Lower binder content increases hardness at the expense of toughness. In any case, check the chemical compatibility of nickel and cobalt when using tungsten carbide in strong acids, as these chemicals can attack binders, causing premature seal failure.
Because tungsten carbide has the highest tensile strength of any popular seal ring material, it's applied routinely in applications that involve high torque and vibration. It's also used in high-pressure and large-diameter applications because its high modulus of elasticity reduces seal face distortion.
Tungsten carbide often is mated with silicon carbide in applications such as heat transfer oils, which would blister carbon graphite. Using two tungsten carbide faces as the inboard set in a dual seal arrangement requires a pumping ring to remove the large amount of frictional heat.
Table 1. Seal ring comparisons. Ratings are for comparison only. Values are relative, with 10 being the highest and 1 being the lowest. They do not reflect magnitudes of difference.
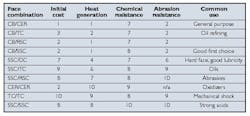
Table 2. Seal ring combination comparisons. Ratings are for comparison only. Values are relative, with 10 being the highest and 1 being the lowest. They do not reflect magnitudes of difference.
Silicon carbide
Silicon carbide is a crystalline material formed by fusing silica and coke at high temperatures. Two forms of silicon carbide used in mechanical seals include reaction-bonded and sintered. Silicon carbide seal rings are particularly well suited to abrasive and corrosive applications and a range of temperature extremes. They are excellent thermal conductors and can be specified when high strength and stiffness are required. Silicon carbide approaches the hardness of diamond and possesses outstanding abrasion resistance.
Reaction-bonded silicon carbide
This fine-grain form of silicon carbide has a matrix of silicon carbide powder and free carbon that has been infiltrated with molten silicon. The molten silicon reacts with most of the free carbon to form silicon carbide, but leaves between 10 percent and 12 percent of the silicon unreacted, which provides some inherent lubrication to decrease wear and heat generation.
However, the presence of free silicon prevents its widespread use in many process industries. Strong acids and caustics attack free silicon, causing premature failure. A common source of chemical attack is caustic washes.
Sintered silicon carbide
This form is the hardest silicon carbide and the most chemical-resistant seal material available. Its hardness, chemical resistance and wear properties are unmatched. It features granular high-density alpha-silicon carbide, molded and sintered at high temperatures to form a solid part. Unlike other seal ring materials, sintered silicon carbide has no free graphite or silicon.
It's not as tough as other seal face materials, however, and it's important that seal designers take this into account. Sintered silicon carbide seal rings should be driven by flat drive pads, not dowel pins, to reduce stress concentrations. In addition, transmitting torque and axial loading using compliant vibration isolation pads minimizes impact stresses. Sintered silicon carbide is increasing in popularity in seal designs that minimize harmful stresses to ensure reliability.
Duplex carbide
Duplex carbide is a reaction-bonded composite of alpha-silicon carbide and graphite. This material is similar to reaction-bonded silicon carbide, except that graphite takes the place of free carbon. Bonding a silicon carbide matrix with graphite and infiltrating it with molten silicon produces duplex carbide.
The material provides the chemical and abrasive characteristics of silicon carbide with the lubricating qualities and lower heat generation of carbon graphite. It contains approximately 70 percent reaction-bonded silicon carbide (6 percent free silicon) and 30 percent graphite. This results in a balance of lubricity and hardness. With its built-in graphite lubricant, it's ideal for use with marginally lubricating fluids. It allows a slightly thicker fluid film between the mating faces, which often is needed for hot water and solvents, where slip-stick and flashing can occur.
Scott Boyson is a manager with A. W. Chesterton Co. He can be reached at (781) 481-6474, [email protected].